
Drug Stability Testing: What It Is, Why It Matters, and Regulatory Requirements
Table of Contents Drug stability testing is one of those areas where the science, the regulations, and real-world use all meet. At its core,
Prompt Praxis Laboratories provides a full range of rapid testing and support services focusing on pharmaceutical products and compounded sterile or nonsterile preparations.
Share This!

Table of Contents Drug stability testing is one of those areas where the science, the regulations, and real-world use all meet. At its core,

Table of Contents Analytical testing labs play a vital role in modern research, manufacturing, and medicine. These specialized facilities evaluate the composition, quality, and


Prompt Praxis Labs is sponsoring and attending the Compounding Pharmacy Compliance 2024, June 18-19, in Boston, MA.

Drug diversion testing is a crucial aspect of healthcare that ensures patient safety and the proper use of controlled substances. Drug diversion refers to the

When it comes to pharmaceuticals, ensuring quality and safety is of paramount importance. One crucial aspect of this process is sterility testing. In this article,

Regular quality control product testing is a critical aspect of ensuring the overall quality and reliability of products. From electronics to pharmaceuticals, conducting frequent testing

Prompt Praxis Labs is sponsoring and attending the Compounding Pharmacy Compliance Congress 2023, August 17-18, 2023 in Boston, MA.

Organizational Readiness for an FDA Audit of a 503B Compounding Pharmacy Webinar, May 4th, 2023 @11 a.m. PT, 2 p.m. ET.
Are you looking for ways to optimize your laboratory’s performance? Discover how gap analysis can help you identify gaps and streamline processes.

Remedial validation is a critical step in data analysis that ensures the accuracy and reliability of your findings. Discover its significance and best practices in

Preformulation support testing is an essential tool for drug development. Find out how it can improve your formulation process and lead to better outcomes.

Validated Test Method Licensing is a process where a laboratory or testing facility licenses a validated test method from another laboratory or testing facility. This
Particulate matter (PM) is a term used to describe a mixture of solid particles and liquid droplets found in the air. These particles can come

Globule Size Analysis is a technique used to determine the size distribution of droplets or particles in a liquid or emulsion. It is commonly used

UV/Vis spectroscopy is a technique used to measure the absorption of light by a sample in the ultraviolet and visible regions of the electromagnetic spectrum.

ICP-OES stands for Inductively Coupled Plasma Optical Emission Spectroscopy. It is a technique used to detect and quantify the presence of heavy metals in a
FTIR (Fourier Transform Infrared) Identification Testing is a method of identifying unknown substances by analyzing their infrared spectra. It is a non-destructive technique that can
Optical rotation is a technique used to measure the rotation of polarized light as it passes through a sample. This technique is commonly used in
Osmolality is a measure of the concentration of solutes in a solution. It is the number of particles per kilogram of water and is expressed
Specific gravity is the ratio of the density of a substance to the density of a reference substance. The reference substance is usually water, which

A melting point determination is a process used to determine the temperature at which a solid substance changes from a solid to a liquid state.

Water Vapor Transmission Testing (WVTR) is a method used to measure the rate at which water vapor passes through a material. This test is commonly

Moisture determination is the process of measuring the amount of moisture present in a substance. It is an important step in many industries, including food,

R&D Stability Testing is a process used to determine the shelf life and stability of a product. It involves testing the product under various conditions

Third Party Confirmation Testing is a process where an independent laboratory tests a product to confirm its quality, safety, and compliance with regulations. This type
Heparin is a medication that is used to prevent blood clots from forming in the body. It is a type of anticoagulant that works by

Batch Release Testing is a process that ensures the quality and safety of pharmaceutical products before they are released to the market. It involves a
ICH Compliant Stability Studies are a set of guidelines established by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)

CMC Support is a service that provides assistance to individuals and organizations in navigating the complex world of clinical research and regulatory affairs.

The first step in selecting a container closure is to consider the product that will be stored inside. Different products have different requirements for container

Compatibility studies are a type of research that evaluates the compatibility of different materials or substances. These studies are commonly used in the pharmaceutical, food,

Hunterlab is a color measurement system that is used to determine the color of various objects. It is a widely used system in industries such

Bacterial Endotoxin Testing (BET) is a process used to detect and quantify the presence of endotoxins in pharmaceuticals, medical devices, and other products.

Before proposing drug product specifications, it is important to consult with regulatory agencies such as the FDA or EMA. These agencies have specific guidelines and

Sterility testing, also known as drug testing, is a process used to detect the presence of drugs, such as cocaine or heroin, in a person’s

At Prompt Praxis Labs, we provide you with the latest and most reliable drug potency testing services. Our laboratory testing is designed to provide an

See how Prompt Praxis Laboratories is impacting the industry in rapid testing, support and validation services! Prompt Praxis Labs focuses on a full range of

Sat, December 3rd @ 11:30 AM CT tune into WPWR-CW, or watch us on Facebook Watch & You Tube. On Sun, December 18 @ 10:30

Analytical Method Development and Validation (AMV) involves the process of creating and testing new analytical techniques to accurately measure components of products.
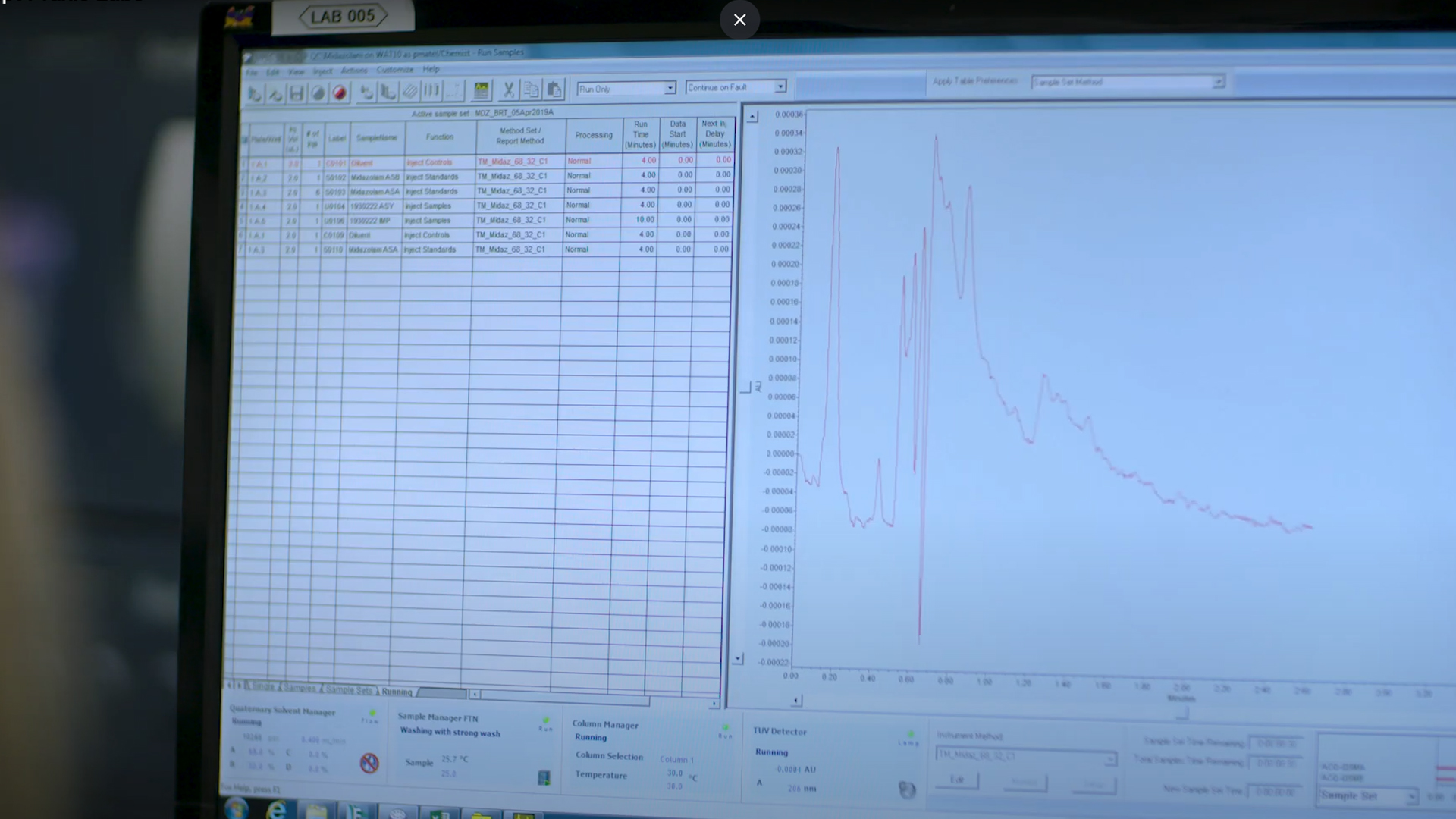
A chromatography column is an important tool for determining what chemicals are in a given sample. You can test any material, such as food, water,

An analytical testing company performs non-clinical (or sometimes referred to as forensic) testing on samples sent in by you or someone else. These labs typically
There are a variety of laboratories you can visit to assess your health and wellbeing. Clinical laboratories, pharmacies, nutritionists’ clinics, and oral surgeons are some

Analytical laboratory testing is a critical process used in the pharmaceutical industry to ensure their products are safe and effective for consumers. These tests, also

Many people are aware of the common diagnostic laboratory tests (e.g., glucose test for diabetes); however, more advanced analytical procedures are now available in the

Recent developments in research labs have made it possible to test almost every product for potential health benefits or risks. Some studies are totally free,

A batch release is an important test that can determine if your skin condition has changed or improved due to treatment. It also determines whether

As your laboratory professional, I will be very direct with you: most laboratories lack consistency in their testing procedures and systems. This is mostly due

There are many ways to test a sample, or a solution, yourself. You can do it with an instrument that requires no external input or

“We want to make sure that patients get the highest quality medicine possible, which means the right molecule at the right concentration with little or